Manufacturing Capability
Flexibility and capacity to meet the needs of your project
Woodstock Sterile Solutions’ manufacturing facility in Woodstock, IL USA, is one of the world’s most comprehensive production environments supporting sterile and non-sterile liquid formulations. Because of our unrivaled experience in the industry, we can provide full-scale development to commercial solutions with customizable specialty fill-finish solutions to ensure a reliable global supply.
With over 30 + production suites and a wide variety of BFS machines, we can handle project sizes from small to very large, including both clinical phase and commercial-scale production. We support a broad range of formulation types, including highly specialized products.
Our manufacturing site features world-class critical utility systems (including water for injection) and cold and frozen storage capabilities.
Our Woodstock facility is staffed and run by one of the industry’s most expert and experienced teams with an outstanding track record of consistent, reliable supply that stretches back decades.
In addition, Woodstock Sterile Solutions is one of the leading contract development and manufacturing (CDMO) providers for sterile liquid formulations. This strong manufacturing orientation ensures your products will be on target and on time.
Ask about taking a Virtual Tour
We are excited to give you the opportunity to check out our manufacturing facility featuring more than 30 blow-fill-seal machines. For more than 50 years, we have partnered with our customers to develop and commercialize products to serve the pharmaceutical and healthcare markets.
We’re looking forward to showing you why Woodstock Sterile Solutions has been recognized as a leading BFS Contract Development and Manufacturing Organization for 50 years. Proven Excellence. Trusted Solutions.
Woodstock Sterile Solutions Manufacturing Facts
- Batch sizes from 5L to 2,000L
- Fill volumes from 0.25mL to 250mL
- Over 30 machines from clinical to high-volume commercial
- Solutions for temperature, light or oxygen-sensitive products
- Support for highly-potent APIs, through category 4
- Sterile and non-sterile manufacturing
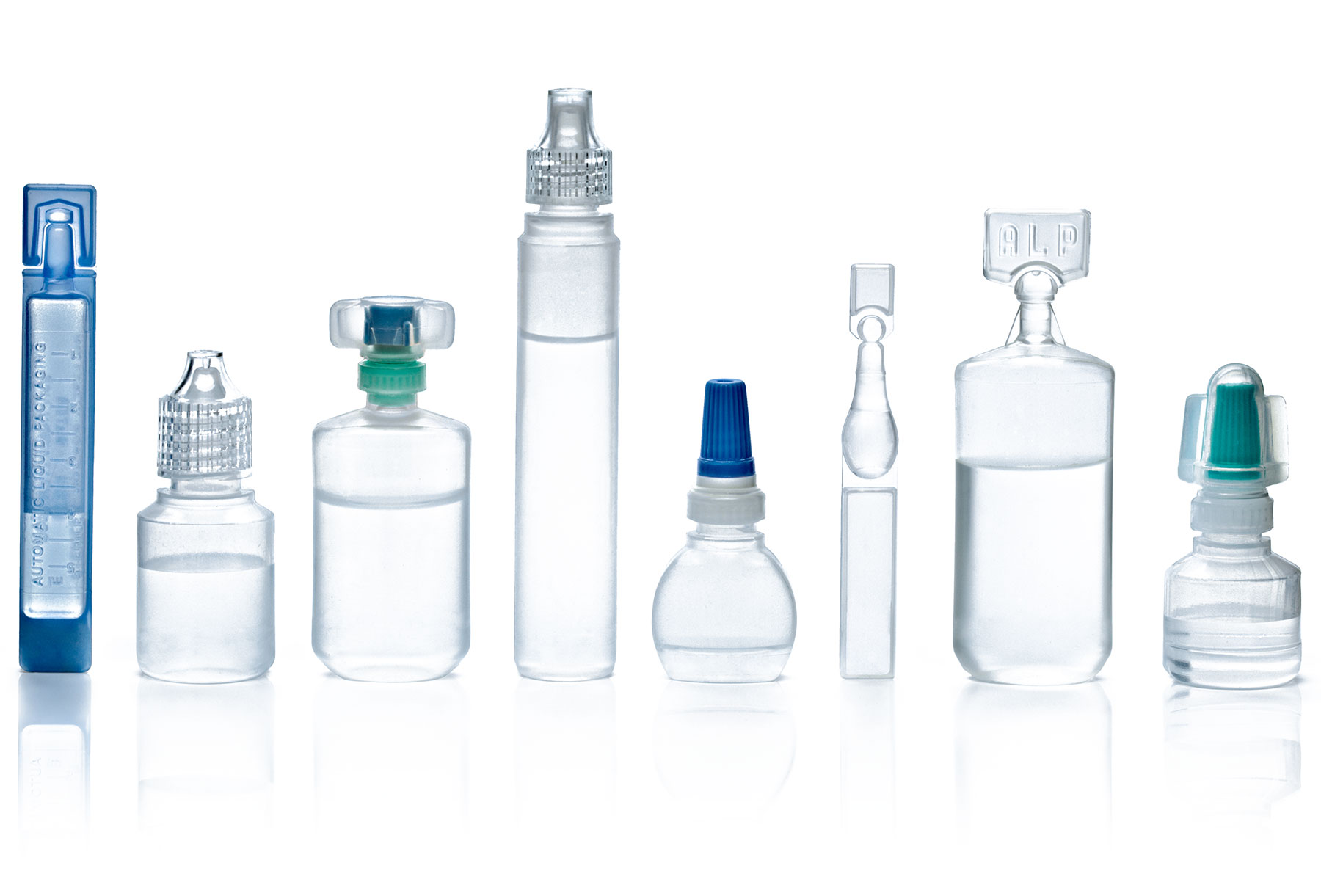
- Unit or multi-dose container options
- Variety of closure options
- Specialized insertion technology
- Standard or unique/customizable container designs
- Various clear and tinted resin options:
- Low-Density Polyethylene (LDPE)
- High-Density Polyethylene (HDPE)
- Polypropylene (PP) resin material
Manufacturing Resources
Article: Mitigating Risks Associated with Aseptic Parenteral Filling
Article: Blow-fill-seal Technology Advances in Aseptic Filling Applications
E-Book: Extractables & Leachables: Understanding Container & Device Interactions
E-Book: BFS Solutions for Inhaled and Biologic Therapies

